Sleeping Without Fear
Study: Artificial pancreas systems with predictive low-glucose-suspend help reduce overnight hypoglycemia in individuals with type 1 diabetes
Bedtime may soon be safer and a lot less anxiety filled for people with type 1 diabetes (T1D) thanks to software being developed as a next-step component for artificial pancreas systems. Called predictive low-glucose-suspend (pLGS), it interacts with a user’s continuous glucose monitor (CGM) and insulin pump to predict when blood sugar is likely to reach a dangerously low threshold and reduce or stop insulin delivery ahead of time in order to prevent life-threatening hypoglycemic—or low blood sugar—episodes.
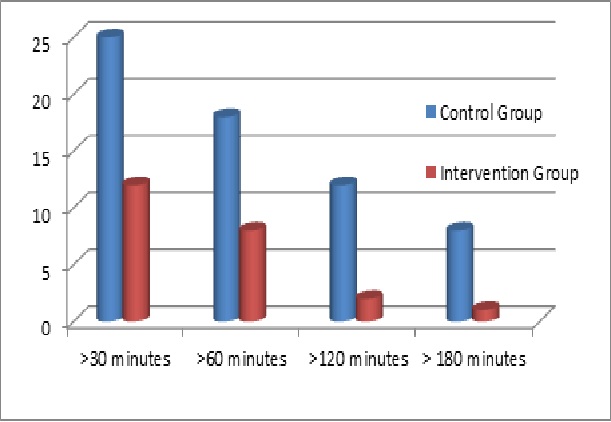
Predictive low-glucose-suspend software is expected to debut in next-generation artificial pancreas systems for sale in Europe later this year and possibly in the United States by next year. In the meantime, researchers across the globe are developing and studying other versions of the technology that may also reach the marketplace, and their findings suggest pLGS-enabled artificial pancreas systems can substantially reduce overnight hypoglycemia. In a recent JDRF-funded home-based study, for example, Stanford University’s Bruce Buckingham, M.D., and David Maahs, M.D., of the Barbara Davis Center for Childhood Diabetes tracked 45 individuals ages 15 to 45 with T1D who tested an investigational artificial pancreas system fitted with their novel pLGS software. Each participant was connected to the system for 42 nights, and investigators randomly activated the pLGS software so that an equal number of nights were spent with it switched on or off. In total the study evaluated 942 nights with the software turned on and 970 nights with it turned off. Participants didn’t know on any given night whether it was switched on or off. Investigators found that participants had far fewer hypoglycemic episodes on nights when the software was active (intervention group) compared to nights when it was inactive (control group), according to findings published in the May 7th online version of Diabetes Care.
Differences in hypoglycemic episodes when trial participants slept without (control group) and with (intervention group) predictive low-glucose-suspend systems
- The vertical axis reflects the percentage of nights that trial participants experienced glucose levels equal to or less than 60 mg/dL
- The horizontal axis reflects the duration of sleep time that trial participants experienced glucose level less than or equal to 60 mg/dL
The study adds to a growing body of research showing that pLGS-enabled systems used during sleep are safe and effective, and the findings help bring people with T1D closer to having fully integrated artificial pancreas systems that offer 24-hour protection against life-threatening hypoglycemic episodes. Following the success of these studies, the researchers are now evaluating the effectiveness of the technology when used by children with T1D as young as three years old.
Artificial pancreas systems are one of JDRF’s priority research areas, and they offer the most immediate promise for improved T1D treatment. To learn more about or to support JDRF’s Artificial Pancreas Program, click here.